Frequently asked Questions
What is an aromatic?
Aromatics are hydrocarbons, organic compounds that consist exclusively of the elements carbon and hydrogen – without which life would not be possible on Earth. Aromatics occur naturally in fossil raw materials such as crude oil and coal, and are produced using feedstocks from steam crackers and refineries.
The main aromatics are benzene, toluene and the xylenes; they are used as starting materials for a wide range of consumer products. Many items taken for granted in our everyday lives rely on products made by the aromatics industry, with benefits like durability, safety, comfort and lightweight design. The items we mean range from the aspirin to the CD – so if you want to know more about the end-uses of aromatics, just check out the section From aromatics to consumer products.
What is the industry doing to reduce the health and environmental impact of aromatics? What are the legal standards the industry has to meet?
The chemical industry as a whole is very strictly regulated. Aromatics producers, in particular, have to comply with a wide body of legislation, both national and European. Governments and the EU have strict regulations on consumer and environmental protection, occupational health, chemical processes and transport and the management of chemical substances. All aromatic substances were registered in 2010 as part of the first Phase of the EU REACH regulation and their registration dossiers are publicly available on ECHA’s dissemination portal. The aromatics industry, via the LOA Consortium remains in regular contact with ECHA and those EU member states involved in overseeing the progression of the registration dossiers through the various REACH evaluation and updates processes.
As well as abiding by existing legislation, aromatics producers, and indeed the whole of the chemical industry, are committed to improving its management of chemicals and chemical processes through the worldwide Responsible Care programme, the world-wide chemical industry’s commitment to continuous improvement in all aspects of health, safety and environment performance including openness in communication about its activities and achievements.
Product Stewardship is part of this initiative, and allows to monitor and then manage products’ impact on health and environment from the moment it leaves the producer’s door to the time it is delivered, with strict and detailed instructions of safe use, to the customer.
We all want chemicals to be as harmless as possible to the environment and our own health. For this reason, the chemical industry has long sought to ensure that its products are safe. Like the rest of society, we want to be satisfied that chemicals, properly used, are as risk-free as possible.
If you want to know more about the efforts of the industry to improve its management of chemicals and chemical processes, click here. You might also want to learn about the international “Long-range Research Initiative”, which funds independent research into the interaction between chemicals, human health and the environment.
Why are aromatics not replaced with other, no-risk products?
Replacing aromatics (benzene, toluene, xylenes) today would not be wise, because there are no substitutes known to date and benzene is still essential for the synthesis of the various products derived from it. Furthermore, aromatics are natural products, and their proprieties and associated risks are known, and have been – and still are – extensively studied. Before proposing the adoption of possible substitutions, one should remember that new products have not been as extensively studied and tested as those that have been used for decades – or centuries.
Very few human activities are entirely devoid of hazard; it is by taking common-sense precautions that we minimise the risk they involve – e.g. by looking left and right before crossing the street. Similarly, common sense tells us that zero-risk products, whether natural or man-made, do not exist. Even common, over-the-counter pain-killers, for example, have to be taken following strict precautions, such as not exceeding a certain dose, or not ingesting them on an empty stomach. This is what “managing the risks” means.
Complying with the control measures protecting consumers and workers, detailed in other parts of this FAQ, allows avoiding any undue risks to employees or the general public.
Are consumers exposed to benzene?
The general public is commonly not exposed to benzene, except for extremely minute amounts from a variety of sources, such as city traffic, open fires and smoking (both active and passive), car refuelling and travelling in a vehicle. Trace amounts of benzene are also detectable in food, as a result of certain type of cooking (e.g. barbecue and grilling).
The overall exposure of an individual to benzene is unique to that individual, since it is dependent on his/her life-style and daily activities, as well as the levels of benzene exposure associated with each of these. In rural areas, for the reasons explained above, exposures are far lower than those found in the cities.
As for the benzene produced by our members, i.e. aromatics producers in Europe, it should be remembered that it is not a consumer product. All the benzene produced by our members is transformed into other chemicals in the processes described above. Once converted into consumer goods the amount of residual benzene is virtually non-existent, as is indeed requested by international regulations, which prohibit the sale of consumer goods containing benzene.
Furthermore, all products derived from benzene are very strictly monitored and regulated by the regulating authorities, who can count on the collaboration of the industry itself, which usually applies even more stringent standards to protect the consumer from any risk.
Are there risks for the health?
Long-term exposure to high levels of benzene vapour in the workplace has been associated with damage to the bone marrow and a low incidence of a specific form of blood cancer. According to our current state of knowledge, today’s controls and working practices provide good protection against the development of these diseases.
The amount of benzene in the open air is many times lower than the high levels just mentioned, but its presence is nevertheless closely monitored.
It should be remembered that zero-risk products, whether natural or man-made, do not exist. Even common, over-the-counter pain-killers, for example, have to be taken following strict precautions, such as not exceeding a certain dose, or not ingesting them on an empty stomach. This is what “managing the risks” means.
Do workers run risks when handling benzene?
Rules for protecting workers handling benzene are extremely strict.
When assessing the health risk of a substance, it is important to consider two criteria: the level of exposure and the degree of hazard. In the case of benzene, the rules for working places are very strict. Because benzene is volatile, inhalation is the major route of exposure. National and international standards seek to limit this exposure by imposing strict occupational exposure limits; the European Commission imposes a limit of 1 ppm (“part per million”, or, in other words, 3.25 milligrams per cubic meter). Industry standards are usually more stringent than this, and often include occupational monitoring programmes to confirm that workplace controls and practices are as safe as possible.
Is benzene transportation safe?
Benzene transport is mainly carried out by sea or inland waterways, and is subject to a number of international guidelines for safe handling of cargoes. In Europe and the USA, ships used for benzene transport meet rigorous standards that exceed those prescribed by the European or American authorities. This forms part of our voluntary commitment to the responsible handling and transport of benzene.
For more details on the regulations governing chemicals transport, and indeed on the industry initiatives to minimise all risks related to it, click here.
What is benzene used for?
Benzene is a very important raw material (intermediate) for the manufacture of a large number of other chemicals (intermediates), used in turn to produce a wide range of products. These include common consumer goods such as pharmaceuticals, TV casings, personal computers, refrigerators, tyres, draining asphalts, food packaging, office and sport equipment, automotive and aircraft components, insulating materials, paints, cosmetics, home care products, etc. That is putting it in a nutshell; for more, see Aromatics in everyday life.
What is benzene?
Benzene is a liquid composed of 6 carbon atoms and 6 hydrogen atoms, configured in a ring shape. It is one of the most important feedstocks for the chemical industry, used for the manufacture of a wide range of everyday items, and is not itself used directly by consumers.
Why is there benzene in gasoline? What about “clean fuels”?
Benzene is a natural component of crude oil, from which gasoline is produced after refining. Industry has taken significant steps to reduce the level of benzene by adapting its formulation of gasoline. Furthermore, industry is upgrading the excess benzene from gasoline into valuable products, such as medical devices, food packaging, auto parts and nylon fibres.
Automotive fuel quality plays an important role in determining the nature and quantity of pollutants emitted by motor vehicles. Cleaner fuels can significantly reduce this source of air pollution.
With the Clean Fuels Directive, the benzene content in the gasoline has been reduced from 5% to 1% as per 1 January 2000, which will have a significant, positive impact on the consumer’s exposure during car refuelling. The European Commission has fixed more stringent standards like the total aromatics content in the gasoline that was reduced to 35% from January 1st 2005. Further reviews have concluded that there was no need to reduce it further.
Are consumers exposed to toluene?
Toluene may be found as a solvent in paints, coating, thinners, inks, detergents, pharmaceuticals etc., and can be released in trace amounts from some plastics. It may be present in the air as a vapour resulting from its use as an octane booster in gasoline and as a solvent.
The overall exposure of an individual to toluene is unique to that individual, since it is dependent on his/her life-style and daily activities, as well as the levels of toluene exposure associated with each of these. In rural areas, exposure due to gasoline is far lower than those found in the cities. Also, when a room is freshly painted with paint containing solvents, the exposure in the room is greater than in pristine air, which is why freshly painted rooms should always be carefully aired.
However, it should be remembered that all products containing toluene are regulated by authorities in collaboration with the industry, so as to apply stringent standards and protect consumers from any risk. European regulations recommend a workplace exposure limit in the air of 50 ppm.
Are there risks for the health?
At unusually high concentrations or in cases of abuse, toluene can produce central nervous system disorders, as can most solvents, including alcohol. There are conflicting data on reproductive risks in women. Miscarriages have been reported in pregnant women sniffing glues containing toluene. For this reason, toluene is labelled as a reprotoxic substance. However, it should be noted that no reproductive effects have been noted in workers occupationally exposed to toluene alone. According to our current state of knowledge, today’s controls and working practices provide excellent protection against these risks.
It should be remembered that zero-risk products, whether natural or man-made, do not exist. Even common, over-the-counter pain-killers, for example, have to be taken following strict precautions, such as not exceeding a certain dose, or not ingesting them on an empty stomach. This is what “managing the risks” means.
Do workers run risks when handling toluene?
According to our current state of knowledge, today’s very stringent “occupational exposure” limits, controls and working practices provide excellent protection against any risks associated to potential exposure to toluene.
Exposure is possible in industries where toluene and gasoline are produced (chemical industry and mineral oil and fuel industry) and where toluene is used as chemical agent or used as an ingredient (e.g. polymer, paints lacquer and varnishes, pulp paper and board, textile, processing and chemical industry). exposure to toluene is also possible whenever toluene containing products are used.
When exposure to toluene occurs, it is primarily by inhalation of vapour and liquid aerosols (e.g. by spray painting). National and international authorities limit the exposure by imposing occupational exposure limits. The recommended workplace limit is 50 ppm (8 hour average). In addition industries strive to reduce exposure with the application of engineering controls and protective equipment requirements in addition to continuous training among workers.
Is toluene transportation safe?
Toluene transport is mainly carried out by sea or inland waterways, and is subject to a number of international guidelines for safe handling of cargoes. In Europe and the USA, toluene transport meets rigorous standards that exceed those prescribed by the European or American authorities. This forms part of our voluntary commitment to the responsible handling and transport of aromatics.
For more details on the regulations governing chemicals transport, and indeed on the industry initiatives to minimise all risks related to it, click here
What is toluene used for?
Toluene is an important raw material (intermediate) for the manufacture of a large number of intermediates, such as toluene di-isocyanate, which is used for the production of polyurethanes. Polyurethanes are a set of versatile products which are used to make the foam in furniture, mattresses, car seats, building insulation, coatings for floors and furniture and refrigerators, sports equipment, etc.
Toluene is also found in some solvents, such as some used in paints and glues, in gasoline (as an octane booster) and is also used to produce explosives. This is far from exhaustive; for more, see the section Aromatics in everyday life.
What is toluene?
![]() Toluene is a liquid composed of 7 carbon atoms and 8 hydrogen atoms, configured in a 6 atoms ring shape with a single atom appendix. All substances with a basic ring structure are called aromatic hydrocarbons, as they often have a characteristic, “aromatic” smell.
Toluene is a liquid composed of 7 carbon atoms and 8 hydrogen atoms, configured in a 6 atoms ring shape with a single atom appendix. All substances with a basic ring structure are called aromatic hydrocarbons, as they often have a characteristic, “aromatic” smell.
Why is there toluene in gasoline? What about “clean fuels”?
Toluene is a natural component of crude oil, from which gasoline is produced after refining. Industry is taking significant steps to reduce the level of aromatic compounds, including toluene, in gasoline, by adapting its formulation.
Automotive fuel quality plays an important role in determining the nature and quantity of pollutants emitted by motor vehicles. Cleaner fuels can significantly reduce this source of air pollution.
The European Commission has fixed stringent standards like the total aromatics content in the gasoline that was reduced to 35% from January 1st 2005. Further reviews have concluded that there was no need to reduce it further.
Are consumers exposed to xylenes?
Xylenes are not sold as such as a consumer product, but we can find them in articles such as paints, varnishes, thinners and some adhesives used in the home. They are used as a solvent in printing (e.g. in rotogravure, for the production of illustrated brochures), rubber and leather industries. Along with other solvents xylenes are also used as a cleaning agent. Small amount of xylenes (between 5 and 10%) can be found in airplane fuel and gasoline .
The overall exposure of an individual to xylenes is unique to that individual, since it is dependent on his/her life-style and daily activities, as well as the levels of xylenes exposure associated with each of these. In rural areas, exposure due to gasoline are far lower than those found in the cities, and when a room is freshly painted with paint containing solvents, the exposure in the room is greater than in pristine air, which is why freshly painted rooms should always be carefully aired.
However, it should be remembered that all products in which xylenes are present are regulated by authorities in collaboration with the industry with the aim of applying stringent standards and protecting consumers from any risk.
Are there risks for the health?
The main effects of xylenes on human health are respiratory irritation, gastrointestinal disturbances and narcosis. High level exposure for short period can cause central nervous system depressant reversible effects – similar to the effects of alcohol. Exposures to high levels in the presence of excessive levels of noise may result in hearing loss. The regulation limit is recommended to minimise the potential for eye and upper respiratory tract irritation and should also provide substantial protection from narcosis, gastrointestinal disturbances and chronic effects believed to result from exposure to higher concentrations. According to our current state of knowledge, today’s controls and working practices provide very good protection against the development of these symptoms.
It should be remembered that zero-risk products, whether natural or man-made, do not exist. Even common, over-the-counter pain-killers, for example, have to be taken following strict precautions, such as not exceeding a certain dose, or not ingesting them on an empty stomach. This is what “managing the risks” means.
Do workers run risks when handling xylenes?
According to our current state of knowledge, today’s very stringent “occupational exposure” limits, controls and working practices provide excellent protection against any risks associated to potential exposure to xylenes.
Besides painters and paint industry workers, others who may be exposed to xylenes include biomedical laboratory workers, distillers of xylenes, wood processing plant workers, automobile garage workers, metal workers and furniture refinishers. In these cases, xylenes are present in the air of workplace as vapour and respiration is the major route of absorption. Less frequently xylenes enter the body through the skin following direct contact.
European regulations recommend an exposure limit in the air of 50 ppm (8 hour average)
Is xylenes transportation safe?
Xylenes transport is mainly carried out by sea or inland waterways, but also by truck and rail tanks and is subject to a number of international guidelines for safe handling of cargoes. In Europe and the USA, xylenes transport meets rigorous standards that exceed those prescribed by the European or American authorities. This forms part of our voluntary commitment to the responsible handling and transport of aromatics.
For more details on the regulations governing chemicals transport, and indeed on the industry initiatives to minimise all risks related to it, click here.
What are xylenes used for?
Xylenes are an important raw material (intermediate) for the manufacture of a large number of intermediates, used to produce many items indispensable to our health, safety and comfort. These include common consumer goods such as pharmaceuticals, detergents, solvents, paints, etc. Mixed-xylenes are also the basic raw material to isolate paraxylene (used in PET for bottles and in the production of polyester fibres), metaxylene (used in PET for bottles and in plastics), and orthoxylene (also used in plastics). Xylenes are also found in some solvents, such as some used in paints and glues and can also be found in gasoline .
Of course, that is putting it in a nutshell ; for more, see the section Aromatics in everyday life.
What are xylenes?
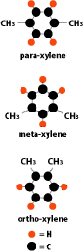 Xylenes are liquids composed of 8 carbon atoms and 10 hydrogen atoms, configured in a 6 atoms ring shape with a two single atom appendixes. Because there are three possible positions in which the two single atoms can be linked, there are three different xylenes which are often mixed when produced (“Mixed xylenes”). All substances with a basic ring structure are called aromatic hydrocarbons, as they often have a characteristic, “aromatic” smell.
Xylenes are liquids composed of 8 carbon atoms and 10 hydrogen atoms, configured in a 6 atoms ring shape with a two single atom appendixes. Because there are three possible positions in which the two single atoms can be linked, there are three different xylenes which are often mixed when produced (“Mixed xylenes”). All substances with a basic ring structure are called aromatic hydrocarbons, as they often have a characteristic, “aromatic” smell.
Why are there xylenes in gasoline ? What about “clean fuels”?
Xylenes are a natural component of crude oil, from which gasoline is produced after refining. Industry is taking significant steps to reduce the level of aromatic compounds by adapting its formulation of gasoline .
Automotive fuel quality plays an important role in determining the nature and quantity of pollutants emitted by motor vehicles. Cleaner fuels can significantly reduce this source of air pollution.
The European Commission has fixed stringent standards like the total aromatics content in the gasoline that was reduced to 35% from January 1st 2005 at latest. Further reviews have concluded that there was no need to reduce it further.
